Researchers headed by a team at Brigham and Women’s Hospital have devised a new way to turn cancer cells into potent anticancer agents. Scientists in the lab of Khalid Shah, PhD, developed a cell therapy approach to eliminate established tumors and induce long-term immunity, training the immune system so that it can prevent cancer from recurring. The team tested their dual-action, cancer-killing vaccine in an advanced mouse model of the deadly brain cancer glioblastoma (GBM), with promising results.
“The team has pursued a simple idea: to take cancer cells and transform them into cancer killers and vaccines,” said Shah, director of the Center for Stem Cell and Translational Immunotherapy (CSTI) and the vice chair of research in the department of neurosurgery at the Brigham and faculty at Harvard Medical School and Harvard Stem Cell Institute (HSCI). “Using gene engineering, we are repurposing cancer cells to develop a therapeutic that kills tumor cells and stimulates the immune system to both destroy primary tumors and prevent cancer.” Shah is corresponding author of the team’s published paper in Science Translational Medicine, which is titled, “Bifunctional cancer cell-based vaccine concomitantly drives direct tumor killing and antitumor immunity,” and in which they concluded, “Here, we developed a bifunctional therapeutic strategy by transforming living tumor cells into a potent agent that concomitantly drives direct tumor killing and antitumor immunity.”
Shah and colleagues have instead devised a different strategy that repurposes living tumor cells, which possess an unusual feature. Like homing pigeons returning to roost, living tumor cells will travel long distances across the brain to return to the site of their fellow tumor cells. Taking advantage of this unique property, Shah’s team used CRISPR-Cas9 gene editing technology to engineer living tumor cells and repurpose them to release a tumor cell killing agent. The engineered tumor cells were in addition designed to express factors that would make them easy for the immune system to spot, tag, and remember, priming the immune system for a long-term antitumor response.
“We first used CRISPR-Cas9 to knock out the IFNβ-specific receptor (IFNAR1) in inherently IFNβ-sensitive syngeneic tumor cells and subsequently engineered them to constitutively produce IFNβ for tumor cell targeting and simultaneous immunomodulation,” they explained. “These therapeutic cells are further designed to coexpress granulocyte macrophage colony-stimulating factor (GM-CSF) that facilitates the differentiation, proliferation, and recruitment of dendritic cells (DCs).”
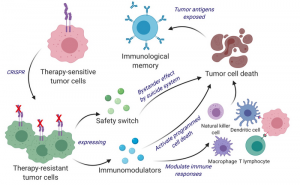
Scientists developed a bifunctional therapeutic strategy by transforming living tumor cells into a therapeutic. Shah’s team engineered living tumor cells using the gene editing tool CRISPR-Cas9 and repurposed them to release a tumor cell killing agent. In addition, the engineered tumor cells were designed to express factors that would make them easy for the immune system to spot, tag, and remember, priming the immune system for a long-term anti-tumor response. The team tested their repurposed CRISPR-enhanced and reverse-engineered therapeutic tumor cells (ThTC) in different mice strains including the one that bore bone marrow, liver, and thymus cells derived from humans, mimicking the human immune microenvironment. Shah’s team also built a two-layered safety switch into the cancer cell, which, when activated, eradicates ThTCs if needed. (Kok Siong Chen and Khalid Shah]
Their experiments demonstrated that the dual-action cell therapy was safe, applicable, and efficacious in the models, suggesting a roadmap toward therapy. While further development and testing will be needed, Shah’s team specifically chose this model and used human cells to smooth the path of translating their findings for patient settings.
“These engineered therapeutic tumor cells (ThTCs) eliminated established glioblastoma tumors in mice by inducing caspase-mediated cancer cell apoptosis, down-regulating cancer-associated fibroblast-expressed platelet-derived growth factor receptor β, and activating antitumor immune cell trafficking and antigen-specific T cell activation signaling,” the investigators stated. “This mechanism-based efficacy of ThTCs translated into a survival benefit and long-term immunity in primary, recurrent, and metastatic cancer models in immunocompetent and humanized mice.”
Shah added, “Throughout all of the work that we do in the Center, even when it is highly technical, we never lose sight of the patient. Our goal is to take an innovative but translatable approach so that we can develop a therapeutic, cancer-killing vaccine that ultimately will have a lasting impact in medicine.”
Shah and colleagues noted that this therapeutic strategy is applicable to a wider range of solid tumors and that further investigation of potential applications is warranted. “Here, we developed a bifunctional therapeutic strategy by transforming living tumor cells into a potent agent that concomitantly drives direct tumor killing and antitumor immunity,” the scientists commented. “Arming naturally neoantigen-rich tumor cells with bifunctional therapeutics represents a promising cell-based immunotherapy for solid tumors and establishes a road map toward clinical translation.”
From Genetic Engineering and Biotechnological News
Share this page