Transmission Zero, a global scientific programme led by scientists at Imperial College London and the Ifakara Health Institute (IHI) of Tanzania, in partnership with the Tanzanian National Institute of Medical Research (NIMR), announces the generation of the first transgenic mosquito strain ever to be made in Africa. This strain carries in its genome genetic modifications that will allow scientists in the future to code for mosquitoes that are unable to transmit malaria. This major scientific achievement is a pivotal milestone in the renewed international efforts to rid Africa of malaria.
The mosquito species that are responsible for the spread of malaria are mainly the Anopheles mosquitoes. These mosquitoes carry the Plasmodium parasite, which is the organism responsible for causing malaria. Mosquitoes can transmit the malaria parasite to humans through bite when they take blood meal. If the mosquito is infected with the malaria parasite, it may also inject the parasite into the human’s bloodstream along with its saliva.
The transmission of the malaria parasite by mosquitoes is a complex process that involves a number of molecular interactions between the parasite, the mosquito, and the host (human). There are several genes in the Anopheles mosquito genome that are associated with the ability of the mosquito to transmit the malaria parasite to humans.
The mosquito saliva contains a complex mixture of proteins, some of which help facilitate blood-feeding and others that aid in the transmission of the malaria parasite. The saliva of Anopheles mosquitoes contains a number of proteins that can interfere with the host’s immune response, which promotes blood-feeding and the survival of the Plasmodium parasite. The mosquito saliva contains anticoagulants, which prevent the blood from clotting, as well as other compounds that help to numb the skin and suppress the host’s immune response.
The gene for salivary gland proteins that are involved in the process of blood-feeding can be genetically interfered with and may not be able to carry the malaria parasite. The genetic modification causes mosquitos to produce compounds in their guts that inhibit parasite growth, making parasites less likely to reach the mosquitos’ salivary glands.
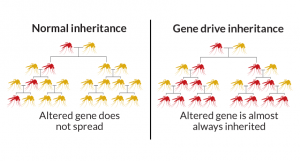
Later, the use gene drive approach in the modified genes for salivary gland proteins in mosquitoes can be introduced to facilitate easy inheritance of resistant genes in mosquito saliva. The gene drive works by altering the inheritance patterns of genes in a way that causes them to spread through a population much more quickly than they would through normal reproduction. This allows the modified resistant trait to be rapidly and efficiently spread throughout the mosquito population and potentially reducing the number of mosquitoes that can transmit the malaria parasite to humans.
Gene drives work by biasing the inheritance process, so the engineered gene is passed on to nearly all offspring, thereby increasing its frequency in the wild much faster than traditional inheritance would allow. This method holds promise for reducing the incidence of diseases transmitted by mosquitoes, but it also raises ecological and ethical concerns that need careful consideration.
The inovation, by researchers from the Transmission: Zero team at Imperial College London in collaboration with Ifakara Health Institute (IHI) and the Tanzanian National Institute of Medical Research (NIMR), designed a ‘gene drive’ technology to spread the modified mosquitos and drastically cut malaria transmission. Gene drives have been proposed as a possible tool for vector control, which promotes the management of organisms that transmit diseases to humans and other animals. One potential application of gene drives in vector control is to modify the genes of the vectors themselves, such as mosquitoes, in order to reduce their ability to transmit diseases.
The team found this developed technique by the Transmission:Zero team could be a powerful tool for bringing down cases of malaria even where transmission is high. The technique has been shown to significantly reduce the possibility of malaria spread in a lab setting, but if proven safe and effective in real-world settings, it could offer a powerful new tool to aid in the elimination of malaria.
Currently, half of the world’s population is at risk of contracting malaria, a disease caused by parasites that are transmitted from one person to another through mosquito bites. In 2021 alone, there were over 247 million cases and 619,000 deaths from malaria, mostly children under five years old in sub-Saharan Africa. With current measures failing to halt disease transmission, the vision of Transmission Zero to modify mosquitoes so that they are unable to transmit the disease could be game changing.
Malaria can have an indirect effect on agriculture, particularly in areas where it is endemic like in sub-Saharan Africa. The agricultural sector is particularly vulnerable to the effects of malaria because it is often labor-intensive, requiring substantial physical exertion. Malaria is a significant health problem in many agricultural areas, and its impact on the workforce can have pronounced effects on agricultural production.
Malaria can cause significant illness and even death, reducing the productivity of agricultural workers and leading to economic losses. The eradication of malaria could significantly have indirect effects on agriculture by improving the health and well-being of people in affected regions. Reducing the incidence of malaria through mosquito control measures can help to keep workers healthy and reduce the economic impact of the disease. However, by reducing the burden of malaria, people may be better able to work and produce, which could have positive effects on agricultural productivity and food security.
Furthermore, it is important to note that genetic modification technology is being increasingly used in agriculture to improve crop yields, reduce the use of pesticides, and make crops more resistant to pests and environmental stresses. Therefore, the development of Gene Drives techniques for mosquito control could lead to a great impact on agriculture production. Apart from human health, these advancements could contribute to more effective pest control, improved production traits, environmental conservation, sustainable agriculture practices, and enhanced food security.
From the Department of Animal, Aquaculture, and Range Sciences in the College of Agriculture, SUA, Morogoro, Tanzania.
Share this page